Peter Paschka and Hartmut Döhner contributed equally.
Background. SARS-CoV-2 infection (COVID-19) has had a great impact worldwide and its mortality has been reported to be higher in patients with haematological malignancies. However, description of its effects and outcomes among recipient of hematopoietic stem cell transplantation (HSCT) is scarce.
Objectives. To describe the characteristics, treatment and outcome of COVID-19 in recipients of HSCT reported to the Madrid registry of COVID-19 ("HEMATO-MADRID COVID-19 registry").
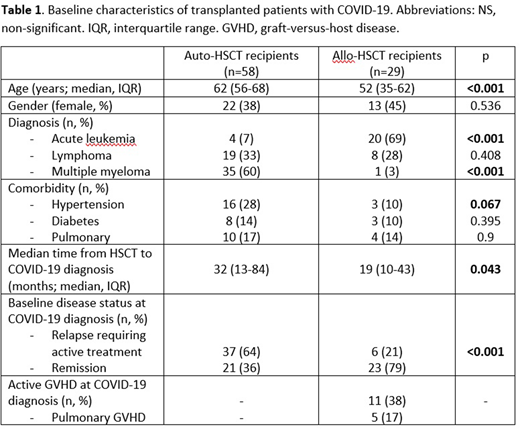
Results. Data of 842 patients from 23 hospitals with haematological malignancies and COVID-19 infection were reported in the Madrid registry between March and June 2020. Among those, 87 (10.3%) patients were HSCT recipients: 58 auto-HSCT and 29 allo-HSCT (7 of them from matched related donor (MRD), 12 matched unrelated donor (MUD) and 10 haplo-HSCT). Characteristics of the population are described in Table 1. Median age at COVID-19 infection was 61 years (IQR, 53-67) and 35 patients (40%) were female. Recipients of auto-HSCT with COVID-19 were older and showed a trend towards a higher incidence of arterial hypertension (28% vs 10%, p=0.067) without statistical differences in other comorbidities; active disease requiring treatment at COVID-19 diagnosis was more frequent in auto-HSCT recipients (65% vs. 21%, p<0.001). Median time from transplant to COVID19 infection was shorter in allo-HSCT patients (32 vs. 19 months, p=0.043). Nearly 40% of allo-recipients had active GVHD at COVID-19 debut, including pulmonary GVHD. A total of 63 (72%) patients required hospital admission and 13 patients (15%) received intensive care support. Allo-recipients showed a trend towards a higher need of hospital admission (79% vs. 68%, p=0.081). Most patients received anti-viral treatment with either hydroxichloroquine (47 patients, 54%), lopinavir/ritonavir (34 patients, 39%), remdesivir (5, 4%) and/or other treatments including azythromicin or interferon. 23 patients (26%) received anticytokine treatment with tocilizumab (15, 17%) and/or anakinra (8, 9%) and 27 patients (31%) received steroids. The overall response rate to treatment and supportive care was 80% in both groups. After a median follow-up of 50 days after the infection debut, overall-survival at day 50 was 84% for auto and 82% for allo-HSCT recipients (p=0.915). However, global mortality rate among the complete observation period was significantly higher in allo-recipients (24% vs. 17%, p <0.001).
Conclusion. In our multicentric experience in a high COVID-19 impacted area, the median time of COVID-19 infection presentation was relatively late in transplanted patients, however shorter in allo-transplanted patients. COVID-19 related mortality was high in HSCT recipients, significantly higher in allo-transplanted patients. Factors associated to this higher mortality should be further investigated to promptly identify high-risk patients since the pandemic is still highly active worldwide.
Kwon:Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Jazz: Consultancy, Honoraria. Duarte:Incyte Corporation: Other: Has received speaker and advisor fees. Hernandez-Rivas:Rovi: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees. Jimenez Yuste:NovoNordisk: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Grifols: Honoraria, Research Funding; Bayer: Honoraria; Sobi: Consultancy, Honoraria, Research Funding; CSL: Honoraria; Octapharma: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal